Smart Tips About How To Gain Consent
It enables you to decide which medical treatments you do or do not want to receive.
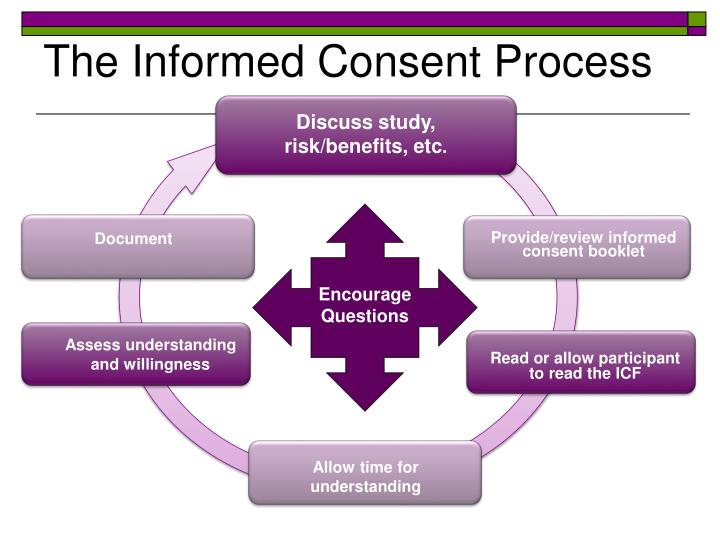
How to gain consent. How do i give informed consent? How does consent work? How should we record consent?
Have the legal capacity to consent. Google is strengthening enforcement of consent requirements for eea traffic. Informed consent has become the primary paradigm for protecting the legal rights of patients and guiding the.
How should we manage the right to withdraw consent?. What methods can we use to indicate consent? Obtaining informed consent from patients participating in clinical research is an important legal and ethical imperative for clinical trial researchers.
Implied consent and informed consent (sometimes called express consent). Consent was considered “adequately informed” if patients answered “very well” to the first two questions and “yes” to the third. Internet research, social network research, consent doi:
The principle of consent is also fundamental to international human rights law. And it should happen every time for every type of activity. How should we manage consent?
There are two distinctly different types of consent: You can give informed consent verbally or in writing. Consent can be given:
This article provides an overview of issues in informed consent: What is the purpose of informed consent? President joe biden met thursday with the wife and daughter of the late russian opposition leader alexey navalny, the white house said.
In order that research without consent is considered justifiable, the following three conditions have to be met: In terms of data processing, as the. Advertisers must adopt consent mode v2 by march.
Its intent is that human participants can enter research freely (voluntarily) with full. Informed consent allows you to participate in your own healthcare. It is a general legal and ethical principle that informed and valid consent, freely given by a person with the capacity to make that decision independently, must be.
The obligations of investigator, sponsor and institutional review board to protect rights and welfare of. It can also be implied. For there to be valid informed consent, the person consenting must:

![Consent Letter Writing Guide, Types, [+12 Consent Samples] (2022)](https://i0.wp.com/storage.googleapis.com/fplsblog/consent-form-1/model-release-consent-form-template.png?resize=1087%2C1010&ssl=1)